General information

Development
Conventional highly immunodeficient mice develop graft-versus-host disease (GvHD) soon after transplantation of Hu-PBMC due to immune attack by human T cells recognizing MHC class I and MHC class II on the tissue surface of mouse cells as foreign bodies. In order to slow down the GvHD response and prolong the experimental window, we deleted IA, H2D and H2K genes on chromosome 17 of NPG mice based on CRISPR-Cas9 technology, and obtained MHC I/II double knockout mice (DK-NPG).
DK-NPG mice combine the characteristics of severe combined immunodeficiency mutation (scid) and Il2rγ gene knockout, possessing a high degree of immunodeficiency. Considering that GvHD is mainly mediated by MHC class I and II molecules, and class I molecules are more widely expressed, the DK-NPG mice have their MHC class I molecules as well as MHC class II molecules knocked out, making it impossible for transplanted Hu-PBMC or isolated T cells and other immune cells to recognize the MHC on the surface of mouse cells, reducing immune attack and slowing down the occurrence of GvHD.
Phenotype
1. The analysis of MHC I/II

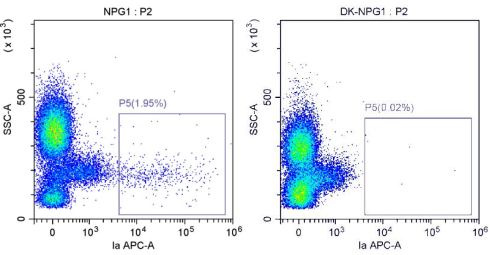
NPG mice DK-NPG mice
Fig 1. The analysis of MHC I and MHC II protein expression in DK-NPG mice by flow cytometry.
Collected peripheral blood from DK-NPG and NPG mice and analyzed the expression of MHC I (mH-2Kd/mH2-Db; left) and MHC II (mI-A; right) by flow cytometry. The results showed that in DK-NPG mice, MHC I and MHC II were not detected, indicating that MHC class I and class II molecules have been completely knocked out.
2. Reconstitution of human PBMC transplanted in DK-NPG mice


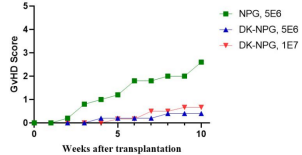
Fig2. Survival, body weight curves, and GVHD scores of NPG and DK-NPG mice inoculated with Hu-PBMC (5×106 vs. 1×107cells/mouse)
After NPG and DK-NPG transplantation with Hu-PBMC cells, DK-NPG mice had a longer survival cycle, steadily gained body weight and exhibited less severe GVHD symptoms (body weight, mobility, posture, hair and skin integrity, etc.) compared to NPG mice.

Fig 3. Immune cell reconstruction efficiency in NPG and DK-NPG mice after inoculation with Hu-PBMC
DK-NPG rebuilt the human immune system well after inoculation with different doses of Hu-PBMC, and the trends were basically the same, indicating that the DK-NPG model stability and rebuilding efficiency were quite high. The reconstruction efficiency of 1E7 Hu-PBMC could reach 60%, and the experimental window could reach 14 weeks; the main reconstructed cells were human CD3 T cells, and the main differentiated hCD3 T cell populations were hCD4 and hCD8; this model could be used to evaluate the function of different CD3 T cell subsets, and to explore their roles in the development of GvHD.
Examples of different tumor modeling and efficacy studies in DK-NPG mice
1. Constructing a human malignant melanoma model and evaluating the therapeutic effect of PD-1 antibody
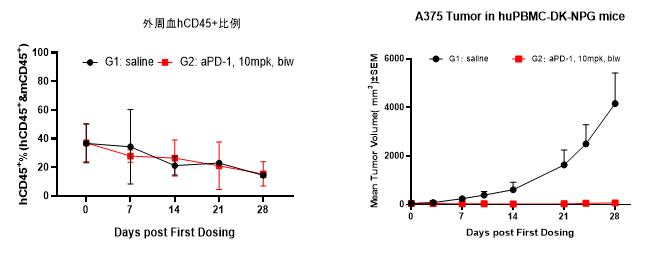
Fig4. Efficacy evaluation of PD-1 antibody in DK-NPG mice after Hu-PBMC reconstruction
Female NPG mice aged 5-6 weeks were inoculated with human PBMCs. One week after reconstitution, A-375 cells were subcutaneously inoculated. When the tumors grew to approximately 100-200 mm3, the mice were randomly divided into two groups (n=6 per group) and treated with PD-1 antibody. The levels of hCD45+ were continuously monitored. The results showed that the PD-1 antibody significantly inhibited the growth of tumor in DK-NPG mice.
2. Constructing a human cervical cancer model and evaluating the therapeutic effect of PD-1 antibody
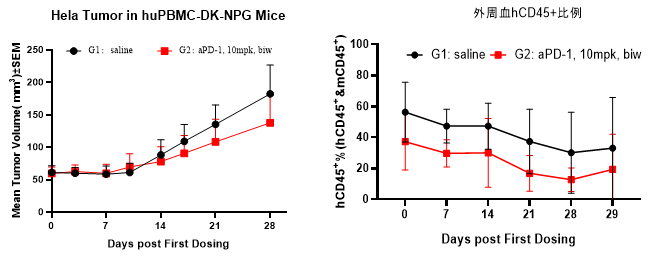
Fig5. Efficacy evaluation of PD-1 antibody in DK-NPG mice after Hu-PBMC reconstruction
5-6 week-old female NPG mice were inoculated with human PBMCs. One week after reconstitution, Hela cells were subcutaneously inoculated. When the tumors grew to approximately 60-80 mm3, the mice were randomly divided into two groups (n=6 per group) and treated with aPD-1 antibody. The levels of hCD45+ were continuously monitored. The results found that the aPD-1 antibody had a certain inhibitory effect on the growth of Hela tumors in DK-NPG mice.
DK-NPG Mice Applications
1. Study the in vivo mechanism of GVHD in xenografts
2. Study of the activation, proliferation and function of CD3 T cells and differentiated CD3 T cell subsets, and exploration of their role in the development of GvHD
3. Pharmacodynamic evaluation, research and development of novel combinations of tumor immunity drugs, CAR-T, TCR-T, small molecule inhibitors, immune checkpoint blockade (ICB) and molecularly-targeted drugs targeting a variety of CD3 T cells, and assessment of the efficacy and safety evaluation of antibody drugs
4. Assessment of treatment-related cytokine release syndrome and evaluation of long-term toxicity related studies of cell therapy
5. Development of new humanized animal models
Reference
1. Ashizawa T, et al. Antitumor Effect of Programmed Death-1 (PD-1) Blockade in Humanized the NOG-MHC Double Knockout Mouse. Clin Cancer Res. 2017, 23(1):149-158.
2. Ashizawa T, et al. Impact of combination therapy with anti-PD-1 blockade and a STAT3 inhibitor on the tumor-infiltrating lymphocyte status. Immunol Lett. 2019, 216:43-50.
3. Santi Suryani Chen, et al. NCG-MHC-dKO mice - an excellent model for PBMC reconstitution and pharmacodynamic evaluation in the absence of GvHD. J Immunol. 2023, 210 (1_Supplement): 89.22.

 animalmodel@vital-bj.com
animalmodel@vital-bj.com +8610-84928167
+8610-84928167